6. 280 g of a mixture containing CA , and C_{2}H_{ 5:2 molar ratio is bumt in presence of excess ofoxygen. Calculate total moles of Co_{2} produced.(A)9 (B)T8 (C)7 (D) 12The | Snapsolve

OneClass: Acetylene gas (C2H2) is burned completely with 20 percent excess air during a steady state-...

Chemical Equations and Reaction Stoichiometry. Chemical equations are used to describe chemical reactions, and they show (1)The substances that react, - ppt download
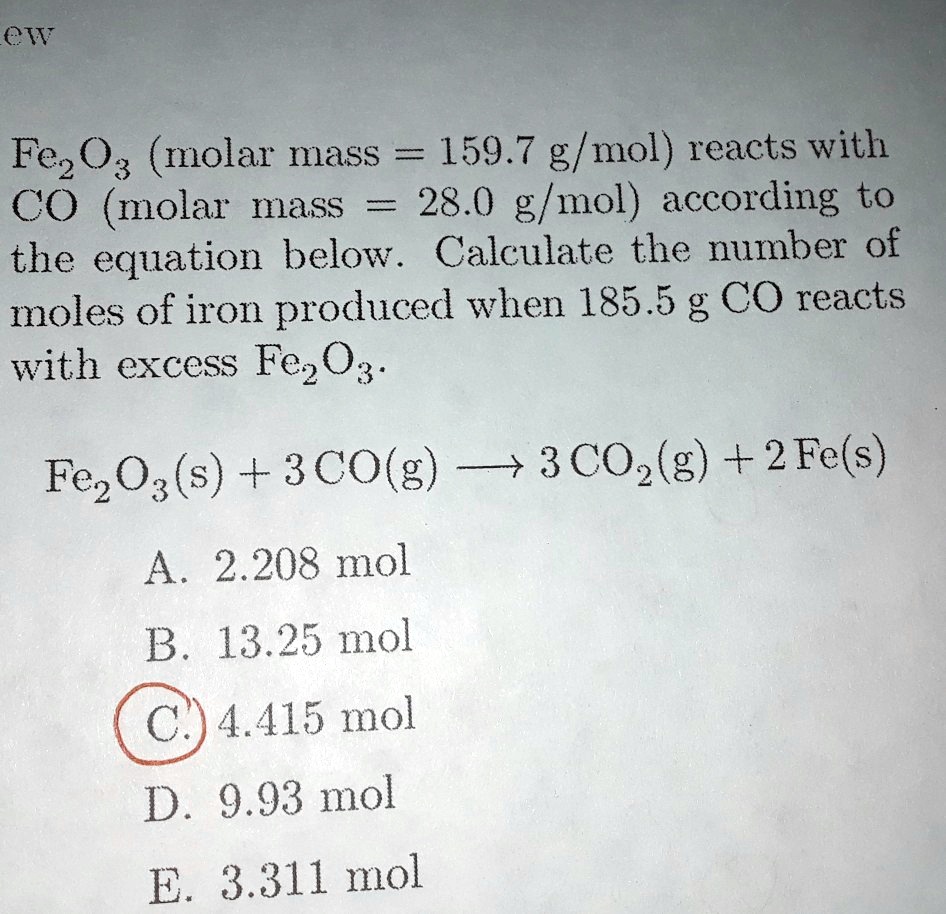
SOLVED: ew Fez O3 (molar mass = 159.7 g/mol) reacts with CO molar mass 28.0 g/mol) according to the equation below. Calculate the number of moles of iron produced when 185.5 g

4. The excess molar Gibbs free energy of formation of solid solutions in the system Au-Cu can be represented by GX = -28280 XauXcu (J) Calculate the activities of Au and Cu

200 mol of ethane are burned in a furnace with 50% excess air. A conversion of 95% is achieved. Calculate the composition of the stack gases?

SOLVED: The molar excess Gibbs free energy of formation of solid solutions in the 'system Au-Ni can be represented by G*s '=XiXAu(QAIAOX Au + 38280X Ni 14230XAuX Ni } 2660 _ Calculate